Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death among women world-wide. Screening for the detection of cervical cancer and pre-cancer has reduced the incidence and mortality rate of this cancer type.
Cervical HPV screening especially for stenotic entry
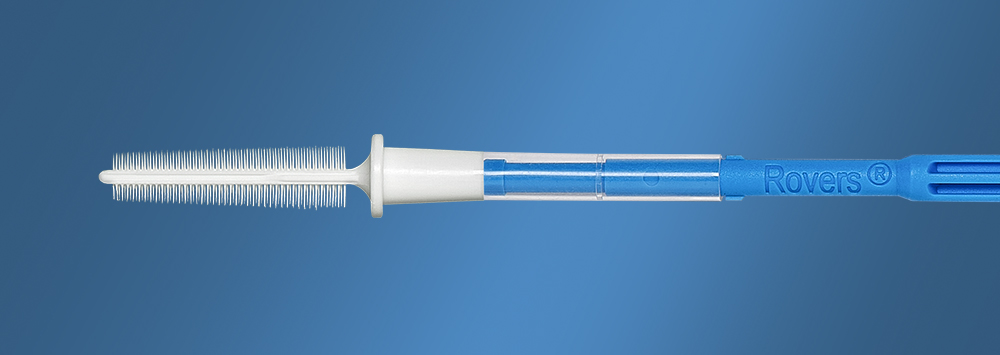
High quality examinations are one of the important elements that determine the efficacy of the screening and has led to the development of a dedicated sampling devices. The EndoCervex-Brush®-S (Small) was developed for endocervical sampling when the entrance of the endocervical canal is narrow or stenotic. The EndoCervex-Brush®-S is developed in collaboration with women and physicians by Rovers Medical Devices, specialist in the development of medical devices for gynaecological examinations for over 20 years.
The EndoCervex-Brush®-S was designed especially for teens, young nulliparous women, or elderly women with a retracted transformation zone and/or stenotic entry of the endocervical canal. The brush provides a stiff yet flexible head with over 246 separate soft, flexible bristles. The bristles constitute a capillary system that facilitates both the collection and release process.
Benefits
- High yield of endocervical cells.
- Female-friendly one piece brush, without rigid traditional wire and nylon bristle combination.
- Reduced cell damage due to the fine bristle technology.
- The stiff yet flexible head with the integrated protective tip gives a reduced chance of tissue damage that has a negative impact on the examination result.
- A safety ring decreases the possibility of the brush to enter into the endometrium during the examination, preventing the collection of endometrium cells.
- Can be used for liquid-based as well as conventional cytology. The hydrophobic material of the brush facilitates the release of the cell material into the fluid.